In this particularly difficult time in human history, it can be hard to figure out what you can do to help, especially if you’re not a medical professional. But as the global race for a viable vaccine to end the pandemic continues, one thing is certain: all candidate vaccines must pass through human clinical trials before it is confirmed viable for mass use.
That’s where you can come in. As clinical trials for a vaccine occur in over a hundred places all over the world, more and more test subjects will be needed. This article will discuss the intricacies of volunteering as a human test subject for COVID-19 vaccine trials, in the event that you would like to make a significant contribution to the world.
How it works
When clinical trials are conducted for any new medicine or treatment, the testing begins with animals. The data gleaned from this testing will then be used to tweak and adjust various aspects of the treatment, like dosage and delivery.
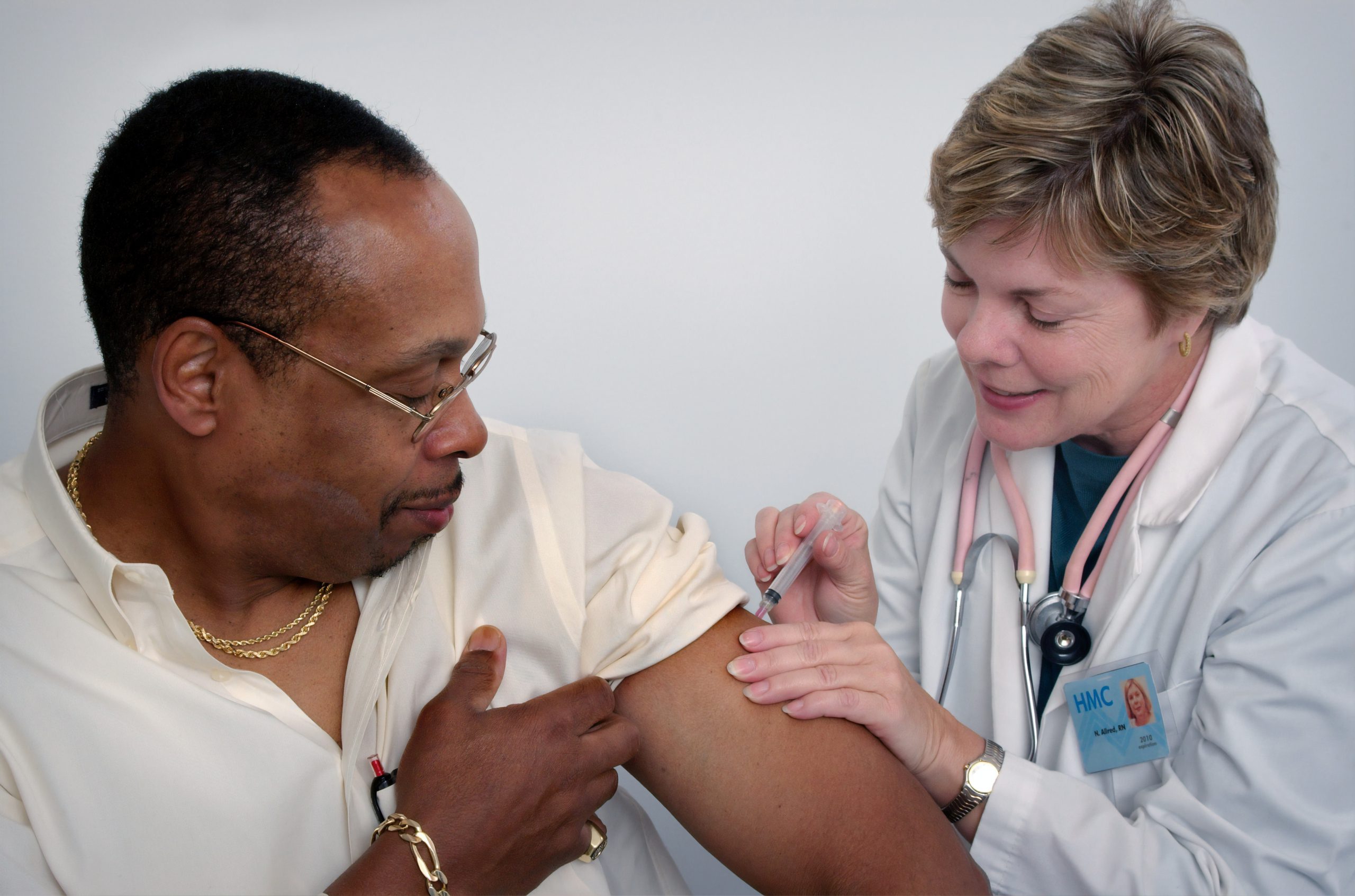
After animal testing, the process then evolves to human trials, using various techniques such as randomization and placebo testing. One group will be inoculated with the vaccine, and the other a harmless solution. Each group will be compared with another to see how much more of each side gets infected than the other.
As these tests must account for any possible long-term side effects, some of them require a commitment of up to two years before the trials conclude, with regular visits involving many medical tests, from drawing blood to receiving injections. Regular communication must also be made between subjects and researchers.
The risks involved
Trials are not without risks, but with vaccine testing, these risks are usually minor. Vaccines are a method of immunization that involves inoculating a patient with a dead or weakened form of a pathogen, in order for the body to build an immunity to the stronger and deadlier versions of the disease.
As such, patients are only expected to experience minor side effects from the vaccine testing. This prototype inoculation will not give recipients the sickness associated with COVID-19 at all.
As gleaned from previous data samplings, roughly 1% will experience a reaction to the vaccine, as the purpose of a vaccine is to generate an immune response. This 1% might experience inflammatory reactions to the vaccine, but likely nothing more serious than that.
How to qualify
It begins with registering at the CoVPN Volunteer Screening Registry website to see if there are any testing sites near you.
Before participating in the study, the informed consent of all the volunteers is obtained to ensure they understand the benefits as well as the risks of being in the study. At any time during the study, a volunteer can leave it should they feel the desire to.
Anyone over 18 years of age can qualify for testing, barring any serious pre-existing conditions, so make sure to think about whether you want to participate and volunteer for COVID-19 vaccine clinical trials.
Conclusion
Now more than ever, every human being needs to think about what they can do for the rest of humanity. Volunteering for COVID-19 is a noble gesture that will be appreciated by millions and millions of people, as it can provide insight that can not only save lives, but improve the quality of life for all 7 billion people around the world.
And if you need more information on COVID-19 vaccines and testing, send us at Dose of Healthcare a message. We have the resources needed to help you protect yourself in this pandemic.